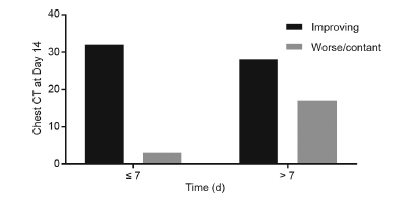
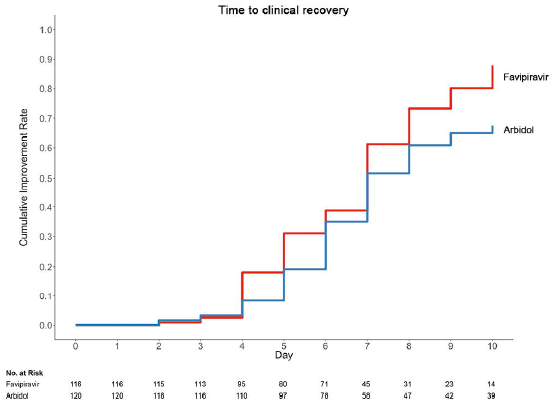
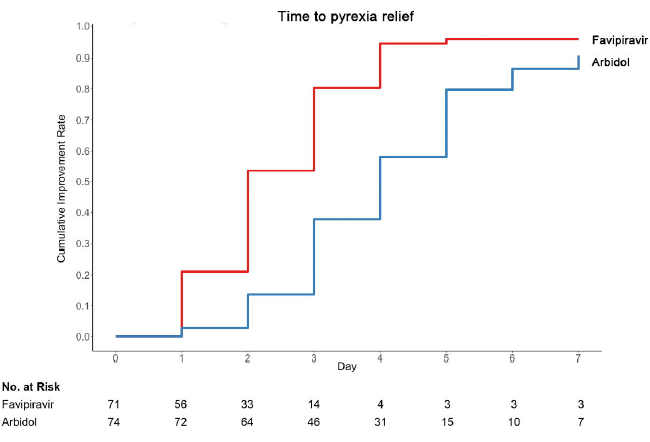
Favipiravir has never been administered with the approved dosage. In clinical studies and the
global phase III study (studies conducted with dose levels lower than the approved dosage),
adverse reactions were observed in 100 of 501 subjects (19.96%) evaluated for the safety
(including abnormal laboratory test values). Major adverse reactions included increase of blood
uric acid level in 24 subjects (4.79%), diarrhoea in 24 subjects (4.79%), decrease of neutrophil
count in 9 subjects (1.80%), increase of AST (GOT) in 9 subjects (1.80%), increase of ALT (GPT)
in 8 subjects (1.60%).
Clinically significant adverse reactions
The following clinically significant adverse reactions have been reported with other similar
anti-influenza virus agents, such as, shock, anaphylaxis, pneumonia, hepatitis fulminant,
hepatic
dysfunction, jaundice, toxic epidermal necrolysis (TEN), oculomucocutaneous syndrome
(Stevens-Johnson syndrome), acute kidney injury, white blood cell count decreased, neutrophil
count decreased, platelet count decreased, neurological and psychiatric symptoms (consciousness
disturbed, abnormal behavior, deliria, hallucination, delusion, convulsion, etc.) and colitis
haemorrhagic.
Patients should be carefully monitored, and if any abnormality is observed, the treatment should
be
discontinued and appropriate measures should be taken.
Other adverse reactions
If the following adverse reactions occur, appropriate measures should be taken according to the
symptoms.
|
≥ 1% |
0.5 - < 1% |
< 0.5% |
| Hypersensitivity |
|
Rash |
Eczema, pruritus |
| Hepatic |
AST (GOT) increased,
ALT (GPT) increased,
γ-GTP increased
|
|
Blood ALP increased, blood bilirubin
increased
|
| Gastrointestinal |
Diarrhoea (4.79%) |
Nausea, vomiting,
Abdominal pain
|
Abdominal discomfort, duodenal ulcer, haematochezia,
gastritis
|
| Hematologic |
Neutrophil count
decreased, white blood cell count
decreased
|
|
White blood cell count increased, reticulocyte count decreased, monocyte
increased
|
| Metabolic disorders |
Blood uric acid increased (4.79%), Blood triglycerides increased |
Glucose urine present |
Blood potassium decreased |
| Respiratory |
|
|
Asthma, oropharyngeal pain, rhinitis, nasopharyngitis |
| Others |
|
|
Blood CK (CPK) increased, blood urine present, tonsil polyp, pigmentation,
dysgeusia, bruise, vision blurred, eye pain, vertigo, supraventricular
extrasystoles
|
Reporting of suspected adverse reactions
Health care professionals, patients/consumers are advised to closely monitor the possibility of the above ADRs associated with the use of favipiravir. If such reactions are encountered, please report to the Hetero either by filling of Suspect Adverse Drug Reactions Reporting Form (form.heteroworld.com) or by Hetero Helpline No.1800-120-8689 and for all India safety cases and complaints, please write to drugsafetyindia@heterodrugs.com.